Cardiovascular diseases are one of the leading causes of death and hospitalization in both genders in nearly all countries of Europe. Besides biochemical or genetic factors, the mechanical properties are crucial for the arteries’ capability to fulfill their physiological function. Therefore, changes of the cyclic wall kinematics as well as wall elasticity are closely related to the origin and progression of cardiovascular diseases such as atherosclerosis, hypertension, heart insufficiency, peripheral vascular disease and aortic aneurysms.
During the past decades, analytical and computational biomechanical models of the human aortic wall, especially of aortic aneurysms, have been developed. In order to be able to solve the direct solid mechanical boundary value problem, i.e. calculate resulting stress, displacement and strain fields in the aortic wall with the required accuracy and reliability, the computational models have to be determined with regard to geometry, material properties and applied loads and boundary conditions. Because of the great inter-individual variance of these biomechanical parameters, patient specific computational models have to be developed if clinical diagnostic use is intended. However, the design of patient individual finite element models remains a challenge since possibilities to acquire patient specific human data in vivo are limited. Most of the approaches presented so far are patient specific only with regard to the geometry of the vessels and blood pressure which is applied as load. In contrast, the constitutive equation of the aortic wall is taken from in vitro experiments or literature. However, the constitutive equation is decisive for the wall kinematics and stresses that will be computed from individual geometry and load.
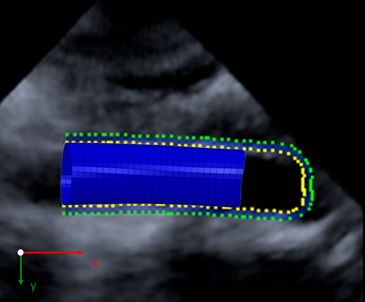
Figure 1: Geometry reconstruction and finite element mesh of a human aortic segment based on time resolved 3D ultrasound data
The aim of this project is to determine individual mechanical properties of the human abdominal aorta based on real time 3D ultrasound measurements combined with speckle tracking (3D wall motion tracking). Human in vivo data are acquired non-invasively in cooperation with the Department on Internal Medicine and Cardiology and the Department of Cardiovascular Surgery, Philipps University Marburg (Germany) and the Department of Vascular and Endovascular Surgery, Goethe University Frankfurt (Germany).
The 3D wall motion tracking data provide the patient individual 3D geometry of an aortic segment (Figure 1) as well as the trajectories of material points on the aortic wall during one pulse cycle.
Figure 2: (A) Systolic displacement vector field plotted on the diastolic reference geometry of the aortic segment. (B) Resulting field of systolic strains. Each point in the scatter plot describes the strain state of one segment of the aortic wall. The strain state is characterized by the combination of longitudinal (ε11) and circumferential (ε22) strains. Each segment has an area of approx. 2 mm2.
On the one hand geometry and measured displacements are used as boundary conditions in finite element simulations that allow the analysis of the wall kinematics throughout the pulse cycle (Figure 2).
On the other hand a nested iterative Finite Element Model Updating (FEMU) workflow is developed in order to solve two coupled inverse problems: a) the determination of the prestrains and prestresses that are present in the imaged wall geometry due to physiological loading and b) constitutive parameter identification of a strain-energy function that models the nonlinear orthotropic hyperelastic material properties of the aortic wall (Figure 3).
The project is carried out in cooperation with the Goethe University Frankfurt am Main, Institute for Cell Biology and Neuro Science (AG Blase). Further cooperation partners are the Departments of Cardiac Surgery and Cardiology, Philipps University Marburg and the Department of Vascular and Endovascular Surgery, Goethe University Frankfurt am Main.
This work was funded by the LOEWE program of the Ministry of Higher Education, Research and the Arts (HMWK, Hesse, Germany) and by the Adolf Messer Foundation.
Figure 3. (A) Identified equibiaxial stress-stretch curves, where σ is the longitudinal and circumferential Cauchy stress, respectively, and λ1 and λ2 are the longitudinal and circumferential principal stretches, respectively. (B) Comparison of measured and estimated systolic circumferential strain distributions.